A Coronavirus outline that has been shared huge number of times is performing exactly how well immunizations against the sickness can work and how we may escape pandemic hell.
Today, counselors to the US Food and Drug Administration casted a ballot for crisis approval for Pfizer’s Coronavirus shot, and the information in this outline is a central explanation why.
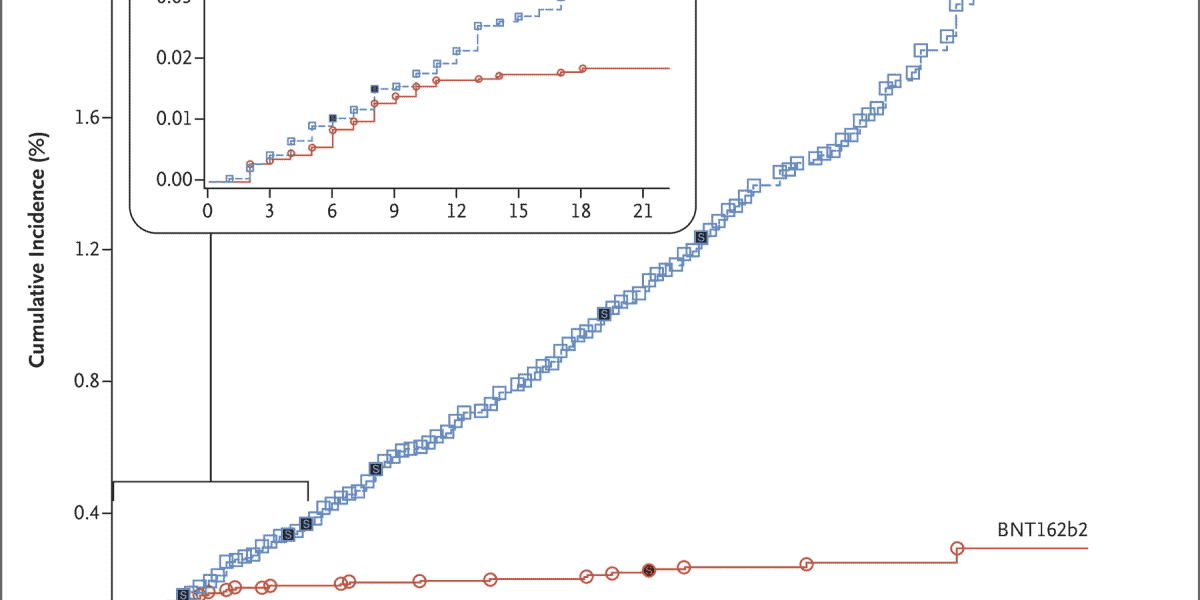
The realistic, delivered by Pfizer and its accomplice, BioNTech, shows the distinction in Coronavirus disease rates between the individuals in their preliminary who got a novel quality antibody and the other people who got a placebo.
The volunteers who were offered a chance of fake treatment show up as the blue line. The ones who got an immunization are in red. Each time either line bounces up, that is the point at which another Coronavirus case occurred.
What the information shows is that during the primary week subsequent to getting their shots, the two gatherings of individuals continued getting Coronavirus at about a similar rate. However, from that point forward, the lines begin to isolate. What’s more, they simply continue isolating, and separating.
That’s the consequence of the immunization producing results, which typically takes a couple of days and gets helped by a subsequent portion. Following fourteen days, barely anybody with the antibody was getting Coronavirus. Yet, the sickness continued striking the individuals who got the fake treatment with precision regularity.
“No remark. This is vaccines main thing,” said Florian Krammer, an unmistakable immunologist, who posted a rendition of the picture to Twitter.
The triumphalism is supported. This is the thing that the specialists have been running after the entire year. Furthermore, the information in this realistic rules out gossipy tidbits, legislative issues, or ignorant analysis. It’s as plain as day: this immunization is outstanding amongst other we’ve seen.
Pfizer introduced the outline in a paper distributed on December 10 in the New England Journal of Medicine and prior in the week as a feature of its application to the US Food and Drug Administration to start selling the antibody. That approval could be given at any second since the office’s consultants have casted a ballot in favor.